Efficacy
The efficacy of PREZCOBIX® in adults with HIV-1 infection is based on efficacy demonstrated in clinical trials of darunavir co-administered with ritonavir [see darunavir full prescribing information].1
ARTEMIS was a randomized, Phase III, open-label, multinational, 192-week (N=689) study in treatment-naïve patients with HIV-1 RNA ≥5000 copies/mL. Participants were randomized to receive either a total daily dose of DRV/r 800/100 mg or LPV/r 800/200 mg. The primary endpoint was to demonstrate noninferiority in virological response of HIV-1 RNA <50 copies/mL at Week 48. One of the secondary endpoints assessed virological response at Week 192.2,3
ODIN was a randomized, phase III, 48-week, open-label (N=590) study to establish noninferiority of once-daily DRV/r 800/100 mg to twice-daily DRV/r 600/100 mg in treatment-experienced patients (with no DRV RAMs at screening) in confirmed virological response (HIV-1 RNA <50 copies/mL) at Week 48.4

In the ARTEMIS trial3:
- At Week 192, the virological response in the ITT population was 68.8% for the DRV/r group and 57.2% for the LPV/r group. The difference in virological response between the treatment groups was 11.6% (95% CI: 4.4 to 18.8).
- 81.3% of the patients in the DRV/r arm with a confirmed virological response of <50 copies/mL at Week 48 maintained that response even at Week 192 vs 68.5% in the LPV/r arm
- Patients in the DRV/r group had a statistically superior virological response compared with those in the LPV/r group (baseline HIV-1 RNA <100,000 copies/mL: 69.5% vs 60.2% [P=0.038; estimated difference in response 9.3%; 95% CI: 0.5; 18.1%], respectively; baseline HIV-1 RNA ≥100,000 copies/mL: 67.5% vs 51.7% [P=0.012; estimated difference in response 15.9%; 95% CI: 3.5; 28.3%], respectively)
- Patients in the DRV/r group with baseline CD4 count ≥200 cells/µL had statistically superior virological response rates vs those in the LPV/r group (71.3% vs 59.6%, respectively; P=0.014; estimated difference in response 11.7%; 95% CI: 2.4; 21.0%)
- Within the subgroup with baseline CD4 counts <200 cells/µL, 65.2% of DRV/r patients achieved HIV-1 RNA <50 copies/mL, compared to 54.1% of LPV/r patients. The difference between groups was not statistically significant for superiority
- A virological response, consistent with the primary endpoint was also observed in the DRV/r arm vs the LPV/r arm across gender, race, region, age and clade subgroups
Resistance
PREZCOBIX®: Has a High Genetic Barrier to Resistance to Help Preserve ARV Susceptibility1
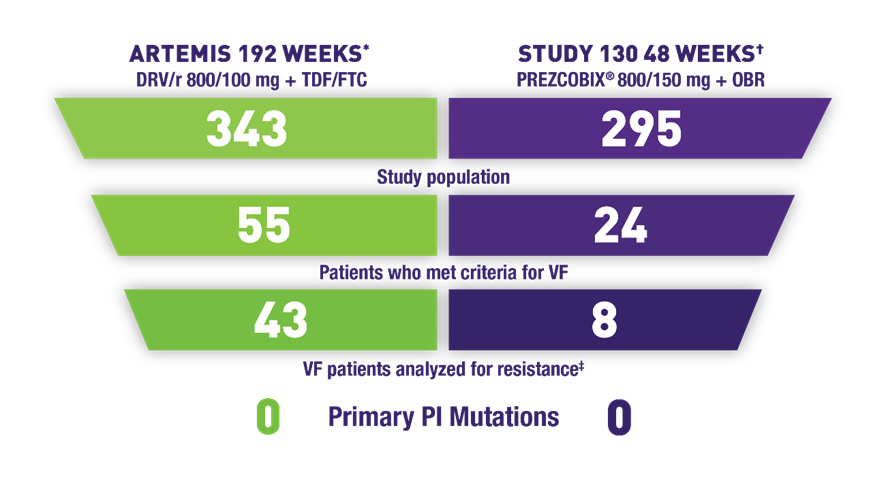
The absence of primary PI mutations does not imply clinical results
- ARTEMIS: 4 patients developed NRTI mutations (M184V)§
- Study 130: 1 patient developed an NRTI mutation (M184V)II
- Zero patients developed the K65R mutation in either study
A low number of treatment-experienced patients developed mutations1
- ODIN¶ 48 weeks, DRV/r 800/100 mg + OBR (n=294): 1 patient developed primary PI mutations (V32I, L76V, I84V)#
- Study 130 48 weeks, PREZCOBIX® + OBR (n=18): 1 patient developed a primary PI mutation (I84I/V); 1 patient developed an NRTI mutation (M184V)
- Zero patients developed the K65R mutation in either study
ARV=antiretroviral; DRV=darunavir; DRV/r=darunavir coadministered with ritonavir; LPR/r=lopinavir/ritonavir; NRTI=nucleoside reverse transcriptase inhibitor; OBR=optimized background regimen; PI=protease inhibitor; RAM=resistance-associated mutation; TDF/FTC=tenofovir disoproxil fumarate/emtricitabine; TE=treatment experienced; TN=treatment naïve; VF=virologic failure.
* Phenotypic data were determined by Antivirogram®. In this test, resistance was defined as a fold change in the 50% effective concentration above the biological/clinical cutoff.
† Study 130: An open-label, single-arm, 48-week, Phase 3 clinical trial evaluating the safety and efficacy of once-daily darunavir, or DRV, 800 mg (2 x 400 mg) + cobicistat, or COBI, 150 mg in HIV-1-infected TN adults (n=295) and TE adults (n=18) with HIV-1 RNA ≥1000 copies/mL and no DRV RAMs (V11I, V32I, L33F, I47V, I50V, I54L, I54M, T74P, L76V, I84V, and L89V). PhenoSense® GT assay; samples with >400 HIV-1 RNA copies/mL were analyzed.
‡ Resistance analysis conducted in patients who had paired genotypic data at baseline and endpoint.
§ None of the subjects had a decreased susceptibility to DRV at virologic failure.
II One TE patient developed a DRV resistance-associated substitution, which was not associated with a decreased susceptibility to DRV. In addition, the patient did not have decreased susceptibility to other PIs. Two patients (one TE and one TN) developed an NRTI resistance-associated substitution, which was associated with a decreased susceptibility to the NRTIs included in their treatment regimens.
¶ ODIN: A randomized, open-label, 48-week, Phase 3 noninferiority clinical trial comparing darunavir 800 mg coadministered with ritonavir 100 mg once daily vs darunavir 600 mg coadministered with 100 mg ritonavir twice daily in TE adult patients with viral load >1000 copies/mL and no DRV RAMs (V11I, V32I, L33F, I47V, I50V, I54L, I54M, T74P, L76V, I84V, and L89V). 60 patients had paired genotypic data at baseline and endpoint.
# One patient, who had previously been treated with lopinavir/ritonavir, developed PI mutations V32I, L76V, and I84V, which in combination were associated with a 24-fold decreased susceptibility to DRV.
PhenoSense GT is a registered trademark of Monogram Biosciences.
Reference: 1. Data on file. Janssen Therapeutics, Division of Janssen Products, LP.
Selected Sections From the DHHS Guidelines:
ART REGIMENS RECOMMENDED FOR MOST PATIENTS LIVING WITH HIV1*†

Boosted DRV with FTC or 3TC plus TAF or TDF‡
- Boosted DRV has a high barrier to resistance and a low rate of treatment-emergent resistance
DTG with FTC or 3TC plus TDF or TAF
- DTG/3TC is not recommended in rapid initiation
BIC/TAF/FTC
- BIC plus TAF plus FTC is recommended as an Alternative ART regimen for pregnant people with early infection and without a history of prior use of CAB-LA as PrEP2
3TC=lamivudine; ART=antiretroviral therapy; BIC=bictegravir; CAB-LA=cabotegravir long-acting injectable; DHHS=Department of Health and Human Services; DRV=darunavir; DRV/c=darunavir/cobicistat; DTG=dolutegravir; FTC=emtricitabine; PrEP=pre-exposure prophylaxis; TAF=tenofovir alafenamide; TDF=tenofovir disoproxil fumarate.
*A sample for genotypic testing should be sent before initiation of ART.1
†For patients who do not have a history of CAB-LA for PrEP.
‡DRV/c is not recommended for use in pregnancy. Utilize perinatal guidelines for use in pregnant patients.1,3
References: 1. Department of Health and Human Services. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Updated September 12, 2024. Accessed November 12, 2024. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-arv/guidelines-adult-adolescent-arv.pdf 2. Carr A, Mackie NE, Paredes R, et al. HIV drug resistance in the era of contemporary antiretroviral therapy: A clinical perspective. Antivir Ther. 2023;28(5). doi:10.1177/13596535231201162 3. SYMTUZA® [package insert]. Titusville, NJ: Janssen Therapeutics, Division of Janssen Products, LP.
Patients at Risk
Do These Patient Factors Impact Your ARV Choice?

Clinical:
- Compliance risk unknown (eg, rapid initiation, newly diagnosed, uncertain adherence)
- With no experience with chronic medication
- Prior low-level viremia
- Associated side effects
- Demonstrated resistance to prior ARV therapy
- No ARV history

Lifestyle/Attitude:
- Busy, irregular schedule (eg, flight attendant, shift work, night classes)
- Psychosocial issues (eg, unstable housing, unemployment, lack of social support)
- Out-of-pocket expenses
- Need for convenience
ARV=antiretroviral.
Reference: Department of Health and Human Services. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Updated September 12, 2024. Accessed November 12, 2024. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/whats-new-guidelines
M184V/I Prevalence
Emerging High-Level Resistance May Impact Future Treatment Options
Emerging DRMs in serial genotype-tested HIV patients (2010-2017):
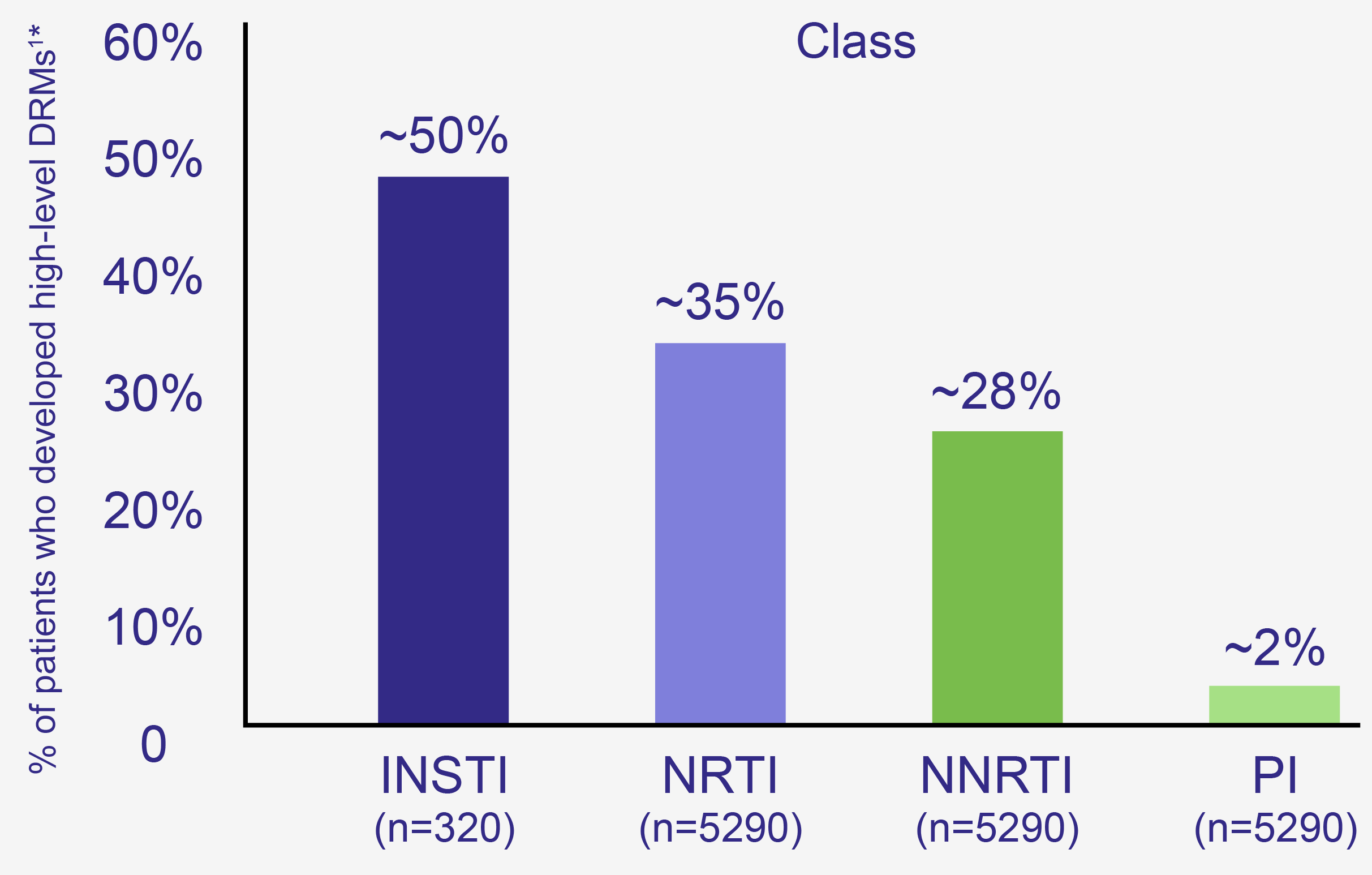
ARV=antiretroviral; DRM=drug resistance mutation; INSTI=integrase strand transfer inhibitor; NNRTI=non-nucleoside reverse transcriptase inhibitor; NRTI=nucleoside reverse transcriptase inhibitor; PI=protease inhibitor; STR=single tablet regimen.
*The presence of drug resistance mutations may not correlate with clinical outcomes.
References: 1. Kagan RM, Dunn KJ, Snell GP, et al. Trends in HIV-1 drug resistance mutations from a U.S. reference laboratory from 2006 to 2017. AIDS Res Hum Retroviruses. 2019;35(8):698-709. doi:10.1089/AID.2019.0063 2. Department of Health and Human Services. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Updated September 12, 2024. Accessed November 12, 2024. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/whats-new-guidelines
SAFETY & TOLERABILITY
Once-daily PREZCOBIX®: Known safety profile with low discontinuation rates due to adverse events1,2
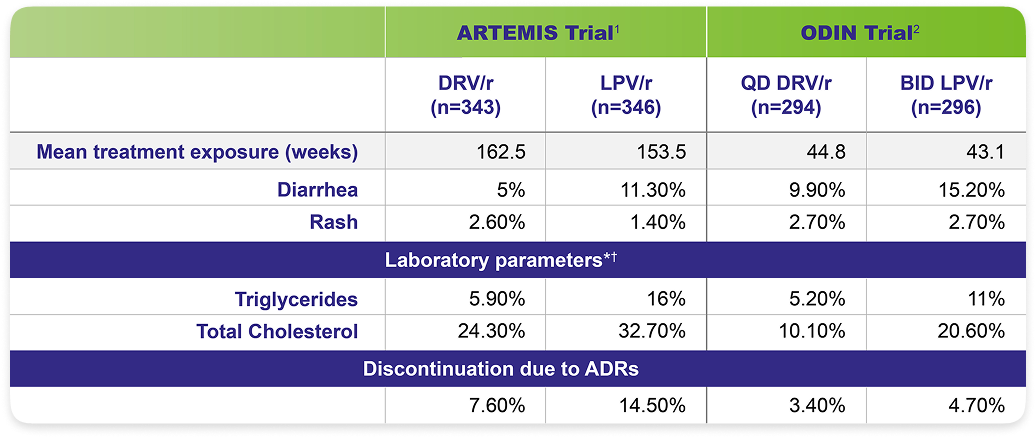
The most frequently reported adverse events considered at least possibly related to treatment (≥5% incidence) were diarrhea, nausea, rash, headache, abdominal pain, and vomiting.2,3
Direct comparisons should not be made between data from separate clinical trials.3
Adverse drug reaction rates observed in clinical trials may not reflect the rates observed in clinical practice.3
*ODIN: Triglycerides [500 mg/dL]. Total cholesterol [240 mg/dL].
†ARTEMIS: Based on the Division of AIDS table for grading the severity of adult and pediatric AEs.
ADRs=adverse drug reactions; ARTEMIS=antiretroviral therapy with TMC114 examined in naïve subjects; BID=twice daily; ODIN=once-daily darunavir in treatment-experienced patients; QD=once daily.
Reference: 1. Orkin C, DeJesus E, Khanlou H, et al. Final 192-week efficacy and safety of once-daily darunavir/ritonavir compared with lopinavir/ritonavir in HIV-1-infected treatment-naïve patients in the ARTEMIS trial. HIV Med. 2013;14(1):49-59. doi:10.1111/j.1468-1293.2012.01060.x 2. Cahn P, Fourie J, Grinsztejn B, et al. Week 48 analysis of once-daily vs. twice-daily darunavir/ritonavir in treatment-experienced HIV-1-infected patients. AIDS. 2011;25(7):929-39. doi:10.1097/QAD.0b013e328345ee95 3. PREZCOBIX® [Prescribing Information]. Titusville, NJ: Janssen Therapeutics, Division of Janssen Products, LP.
DHHS Guidelines: Weight
Selected Sections From the DHHS Guidelines: Weight Gain
ART=antiretroviral therapy; ARV=antiretroviral; BIC=bictegravir; DHHS=Department of Health and Human Services; DTG=dolutegravir; EFV=efavirenz; EVG/c=elvitegravir/cobicistat; INSTl=integrase strand transfer inhibitor; NNRTI=non-nucleoside reverse transcriptase inhibitor; Pl=protease inhibitor; TAF=tenofovir alafenamide; TDF=tenofovir disoproxil fumarate.
References: 1. Department of Health and Human Services. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Accessed November 12, 2024. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-arv/guidelines-adult-adolescent-arv.pdf 2. Chandiwana NC, Siedner MJ, Marconi VC, et al. Weight Gain After HIV Therapy Initiation: Pathophysiology and Implications. J Clin Endocrinol Metab. 2024;109(2):e478-e487. doi:10.1210/clinem/dgad411 3. Koethe JR, Lagathu C, Lake JE, et al. HIV and antiretroviral therapy-related fat alterations. Nat Rev Dis Primers. 2020;6:48. doi:10.1038/s41572-020-0181-1
Patients at Risk: Weight
What Patient Factors Weigh Into Your ARV Choice?
ARV-RELATED WEIGHT CONSIDERATIONS
Demography-related
- Race
- Gender
- BMI
Disease stage
Reflected by low baseline CD4 cell count and high baseline HIV RNA
Treatment-related
Current ART being used
ART=antiretroviral therapy; ARV=antiretroviral; BMI=body mass index; CD4=cluster of differentiation 4; RNA=ribonucleic acid.
Reference: Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: Risk factors in randomized comparative clinical trials. Clin Infect Dis. 2020;71(6):1379-1389. doi:10.1093/cid/ciz999
DOSING
Once-Daily PREZCOBIX®
Coformulated in a fixed-dose combination1
PREZCOBIX® is a two-drug combination of darunavir, a human immunodeficiency virus (HIV-1) protease inhibitor, and cobicistat, a CYP3A inhibitor, and is indicated for the treatment of HIV-1 infection in treatment-naïve and treatment-experienced adults and pediatric patients who1:
- Weigh at least 25 kg
- Have no DRV RAMs*
The recommended dosage of PREZCOBIX® is one tablet taken once daily orally with food in conjunction with other antiretroviral agents.1†‡
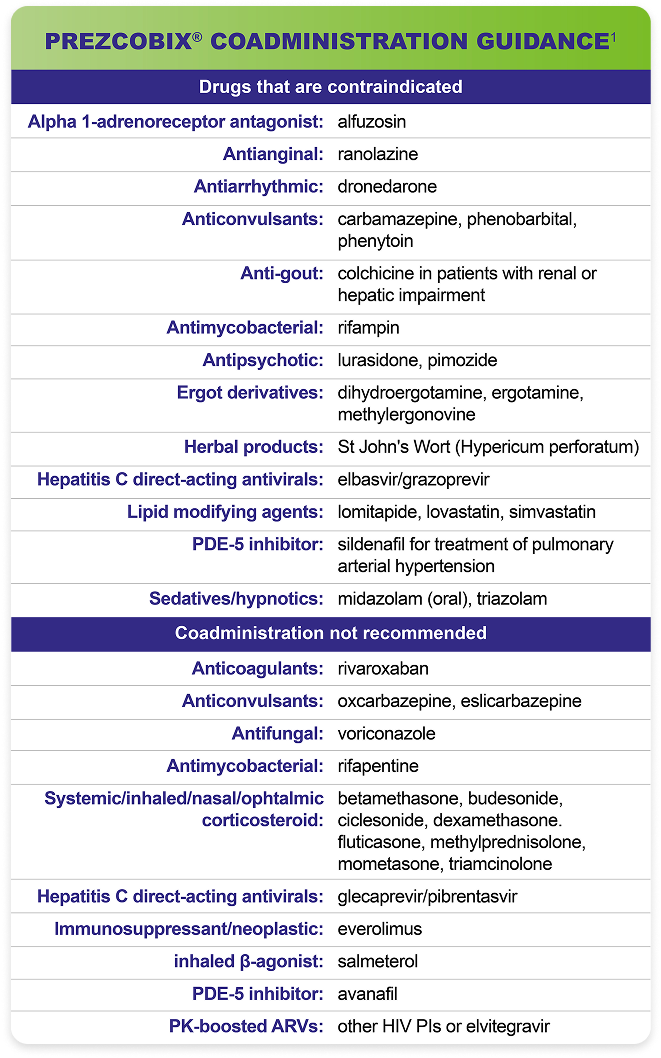
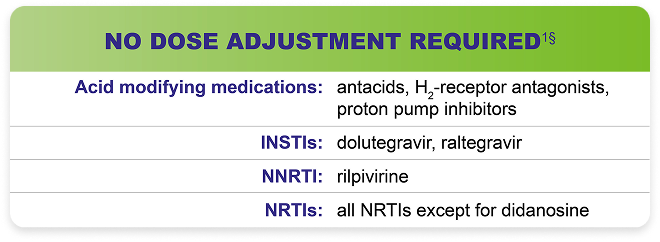
Testing Prior to Initiation of PREZCOBIX®1
HIV Genotypic Testing:
HIV genotypic testing is recommended for antiretroviral treatment-experienced patients. However, when HIV genotypic testing is not feasible, PREZCOBIX® can be used in protease inhibitor-naïve patients, but is not recommended in protease inhibitor-experienced patients.
Creatinine Clearance:
Prior to starting PREZCOBIX®, assess estimated creatinine clearance because cobicistat decreases estimated creatinine clearance due to inhibition of tubular secretion of creatinine without affecting actual renal glomerular function. When co-administering PREZCOBIX® with tenofovir disoproxil fumarate (tenofovir DF), assess estimated creatinine clearance, urine glucose, and urine protein at baseline.
Not Recommended in Severe Renal Impairment1
PREZCOBIX® co-administered with tenofovir DF is not recommended in patients who have an estimated creatinine clearance below 70 mL per minute.
Not Recommended in Severe Hepatic Impairment1
PREZCOBIX® is not recommended for use in patients with severe hepatic impairment.
Not Recommended During Pregnancy1
PREZCOBIX® is not recommended during pregnancy because of substantially lower exposures of darunavir and cobicistat during the second and third trimesters. PREZCOBIX® should not be initiated in pregnant individuals. An alternative regimen is recommended for those who become pregnant during therapy with PREZCOBIX®.
ARV=antiretroviral; CrCl=creatinine clearance; DHHS=Department of Health and Human Services; INSTI=integrase strand transfer inhibitor; HMG-CoA=3-hydroxy-3-methyl-glutaryl-coenzyme A; NNRTI=non-nucleoside reverse transcriptase inhibitor; NRTI=nucleoside reverse transcriptase inhibitor; PDE-5=phosphodiesterase type 5; PI=protease inhibitor; PK=pharmacokinetic; TAF=tenofovir alafenamide; TDF=tenofovir disoproxil fumarate.
*DRV RAMs=darunavir resistance-associated mutations: V11I, V32I,
†L33F, I47V, I50V, I54L, I54M, T74P, L76V, I84V, and L89V.1
‡When PREZCOBIX® is used with TDF/FTC, HIV-1–infected adults must have a CrCl ≥70 mL/min.2
This DHHS recommendation is based on data from comparative trials demonstrating that TAF-containing regimens are as effective in achieving or maintaining virologic suppression as TDF-containing regimens, but with more favorable effects on markers of renal and bone health.2
§No clinically relevant drug-drug interaction is expected when PREZCOBIX® is coadministered.
References: 1. PREZCOBIX® [Prescribing Information]. Titusville, NJ: Janssen Therapeutics, Division of Janssen Products LP.
2. Department of Health & Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Published October 17, 2016. Accessed November 12, 2024. https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf



